Bioretec

1- ActivaPin™ and ActivaNail™
Product Overview

Bioretec’s ActivaPin™ is ideal for fixation of fractures and osteotomies in upper and lower extremities.
ActivaNail™ offers additional support with its small head.
Benefits of using ActivaPin™ and ActivaNail™
Self-Locking SL™ technology and grooved surface design enable pins and nails to stay in place with improved rotational stability. Just choose a suitable pin or nail and tap it quickly into place with Bioretec pin applicator.
The usage of ActivaPin™ gives surgeon more freedom during the surgery.
After insertion scissors, reciprocating saw or a hot wire can be used
to cut the ActivaPin™ if needed.
 | | |
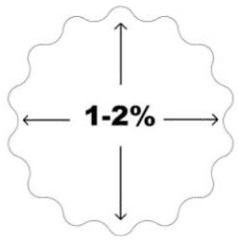
| Image: ActivaPin™ cross section | Image: Material memory effect. Dimensional changes in human body conditions: diameter will increase and length will decrease 1-2% compared to the initial dimensions. * | |
|  | |
| Image: ActivaPin™ Applicator - Unique tip keeps pin place without risk of dropping pin during the insertion. | Image: Packaging, instrument and implant design enables aseptic and easy use in surgical procedure. |
Indication examples
Forefoot
- Ankle fractures.
- Talar fracture.
- Calcaneal fractures.
- Hallux valgus surgery.
- Femoral head fractures.
- Femoral condylar intra-articular fractures.
- Patellar fractures.
- OCD.
Upper extremities
- Distal clavicular fracture.
- Glenoid rim fractures.
- Proximal humeral neck fractures.
- Epiphyseolytic fractures in upper limb in children.
- Intra-articular fractures of humeral capitellum through the articular surface.
- Lateral humeral condyle fractures.
- Medial humeral condyle or epicondyle fractures.
- Olecranon fractures.
- Radial head fractures.
- Distal radial fractures.
- Hand fractures.
Benefits of using ActivaPin™ or ActivaNail™ in fixation of radial head fractures:
- Great benefit of direct transarticular fixation of radial neck or head.
- No protruding implants at the joint surfaces.
- Far less invasive than plate fixation.
- No K-wires through the skin.
- Can be inserted fully arthroscopically.
Material properties
ActivaPin™ gives an excellent fixation enabling bone to maintain its own strength.
ActivaPin™ is made of PLGA** and biodegrades in the body safely and in a controlled manner within approximately two years.
Self-Locking™ technology - the diameter of the implant expand. *
** PLGA - poly(lactic-co-glycolic acid), optimal combination with a long history of safe medical use and the degradation by hydrolysis into alpha-hydroxy acids that are metabolized by the body.
ActivaPin™ and ActivaNail™ are FDA cleared and CE approved.
Ideal for hammertoe fixation
The following video demonstrates the usage of ActivaPin™ in hammertoe fixation.
Product range
ActivaPin™ is available in diameters 1.5, 2.0, 2.7 and 3.2 mm and lengths 20 – 70 mm.
ActivaNail™ is available in diameter 1.5 and lengths 10-30 mm and diameter 2.0 and lengths 15-30 mm.

Product sheets: ActivaPin™, ActivaNail™,
Chevron Technique by using ActivaPin™
Hammertoe Technique by using ActivaPin™
2- ActivaScrew™ Cannulated
Product Overview

Bioretec’s ActivaScrew™ Cannulated offers a broad range of fully threaded and partially threaded cannulated screws for fixation of fractures and osteotomies.
Implants have been used worldwide and the track record is excellent (in US MAUDE database no records). The occurrence of potential complications is less than 0.01%.
Benefits of using ActivaScrew™ Cannulated
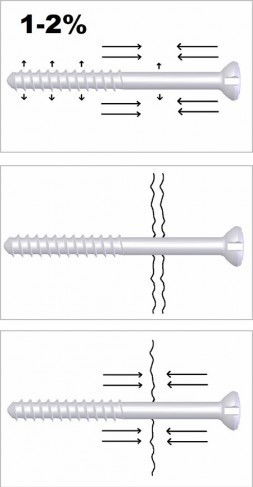
Auto-Compression™ to reduce the risk of unstable fixation. Auto-Compression™ tightens the screw lengthwise contraction of the screw.
Due to Auto-Compression™ Bioretec ActivaScrew™ Cannulated has ability to maintain compression during the bone healing.
The functionality of the ActivaScrew™ Cannulated begins during surgery, actively maintaining fixation during and after implantation, which is conducive to proper ossification.
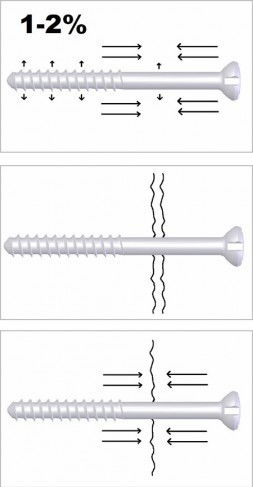 |
| |
| Image: Auto-Compression™ is caused by dimensional changes in hydrolytic conditions. Diameter of the screw will increase and length of the screw will decreased 1 - 2% compared to the initial dimensions.
| ||
| 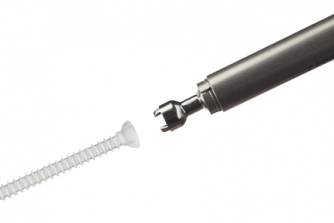 | |
| Image: Packaging, instrument and implant design enables aseptic and easy use in surgical procedure. | Image: Screws are delivered with disposable metallic insertion adapter. |
Indication examples
Lower extremities
- Ankle fractures.
- Talar fractures.
- Calcaneal fractures.
- Hallux valgus surgery (Proximal osteotomy or TMT-I arthrodesis).
- Talocrural arthrodeses.
- Subtalar arthrodeses.
Upper extremities
- Distal clavicular fracture.
- Glenoid rim fractures.
- Proximal humeral neck fractures.
- Epiphyseolytic fractures in upper limb in children.
- Lateral humeral condyle fractures.
- Medial humeral condyle or epicondyle fractures.
- Olecranon fractrures.
- Distal radial fractures.
- Hand fractures.
- Scaphoideus.
Please refer the approved indication in your country.
Material properties
ActivaScrew™ Cannulated gives an excellent fixation enabling bone to maintain its own strength.
ActivaScrew™ Cannulated is made of PLGA** and biodegrades in the body safely and in a controlled manner within approximately two years.
** PLGA - poly(lactic-co-glycolic acid), optimal combination with a long history of safe medical use and the degradation by hydrolysis into alpha-hydroxy acids that are metabolized by the body.
ActivaScrew™ Cannulated is FDA cleared and CE approved.
Bioretec ActivaScrew™ Cannulated: high strength and stiffness facilitate stable fixation.
The following video demonstrates the strength of Bioretec screws.
Product range
ActivaScrew™ Cannulated is available in diameters 3.5 and lengths 20 - 40 mm. In diameters 4.0 and 4.5 lengths 40 – 90 mm.

Product sheet: ActivaScrew™ Cannulated for international market only
3- ActivaScrew™
Product Overview

Bioretec’s ActivaScrew™ is for fixation of bone fractures, osteotomies, arthrodeses, bone grafts and osteochondral fractures of upper extremity, ankle and foot in the presence of appropriate immobilization.
Implants have been used worldwide and the track record is excellent (in US MAUDE database no records). The occurrence of potential complications is less than 0.01%.
Benefits of using ActivaScrew™
Auto-Compression™ to reduce the risk of unstable fixation. Auto-Compression™ tightens the screw lengthwise contraction of the screw. Due to Auto-Compression™ Bioretec ActivaScrew™ has ability to maintain compression during the bone healing
The functionality of the ActivaScrew™ begins during surgery, actively maintaining fixation during and after implantation, which is conducive to proper ossification.
“I have used Bioretec bioabsorbable products since 2008, specially Bioretec ActivaScrew™.
I encountered no screw breakage during the implantation, neither have we seen any cysts or
adverse tissue reactions related to the Bioretec´s implants. I am very pleased with results,”
says Chief Surgeon from Finland.
 |
| |
| Image: Auto-Compression™ is caused by dimensional changes in hydrolytic conditions. Diameter of the screw will increase and length of the screw will decreased 1 - 2% compared to the initial dimensions.
| ||
| 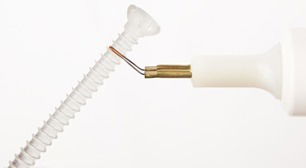 | |
| Image: Packaging, instrument and implant design enables aseptic and easy use in surgical procedure. | Image: Cutting the ActivaScrew™ fully threaded with hot wire. |
Indication examples
Lower extremities
- Ankle fractures
- Talar fractures
- Calcaneal fractures
- Hallux valgus surgery (Proximal osteotomy or TMT-I arthrodesis)
- Talocrural arthrodeses
- Subtalar arthrodeses
Upper extremities
- Distal clavicular fracture
- Glenoid rim fractures
- Proximal humeral neck fractures
- Epiphyseolytic fractures in upper limb in children
- Lateral humeral condyle fractures
- Medial humeral condyle or epicondyle fractures
- Olecranon fractrures
- Distal radial fractures
- Hand fractures
- Scaphoid fractures
Please refer the approved indication in your country.
Material properties
ActivaScrew™ is made of PLGA** and biodegrades in the body safely and in a controlled manner within approximately two years.
** PLGA - poly(lactic-co-glycolic acid), optimal combination with a long history of safe medical use and the degradation by hydrolysis into alpha-hydroxy acids that are metabolized by the body.
ActivaScrew™ is FDA cleared and CE approved.
Bioretec ActivaScrew™ is compatible with AO standards
The following video demonstrates the usage of ActivaScrew™.
Product range
ActivaScrew™ is available in diameters 2.0, 2.7, 3.5 and 4,5 mm and lengths 20 – 90 mm (LAG 20 - 70 mm).

Product sheet: ActivaScrew™ for international market only